Patient recruitment for PredictTB clinical trial expected to restart in July 2020
Patient recruitment for PredictTB clinical trial in South Africa is scheduled to restart within the next month after having been at a halt since the end of March this year. The on-site staffers are already getting ready to welcome back patients by ensuring that everyone has the right personal protective equipment (PPE) to handle both TB and COVID-19 cases. Now, all that is needed to resume activity at the local community health centres is an approval from the Western Cape Department of Health.
Recruitment likely to restart gradually over the next weeks
There are five PredictTB clinical trial sites in South Africa, two tied to the Stellenbosch University’s Ethics Department, and three linked to the University of Cape Town’s Ethics Department. Both universities’ ethics department have confirmed that they are ready to restart recruitment.
The most likely scenario is that recruitment will restart gradually over the next weeks. Bronwyn Smith, Clinical Project Coordinator at Stellenbosch University, predicts that at least one or two sites will be back in business in the next two to four weeks.
The PredictTB team at the Stellenbosch University recently submitted an updated protocol amendment to SAHPRA, South African Health Products Regulatory Authority, in which they introduced COVID-19 testing and made provision for COVID-19 co-incidence in the study procedures and outcome assessment.
However, when sites are ready to take on participants again, recruitment will most likely have to resume on the current, already approved, protocol until SAHPRA grants approval for the new protocol.
Recruitment period prolonged until the end of 2020
When recruitment was paused earlier this year, approximately 85% of the target of enrolling 310 participants into the shortened treatment arm B/C (total of 620 patients for all arms of the study) divided between China and South Africa had been reached. Recruitment was initially scheduled to end this June. However, the recruitment period has now been prolonged and is likely to run until the end of the year.
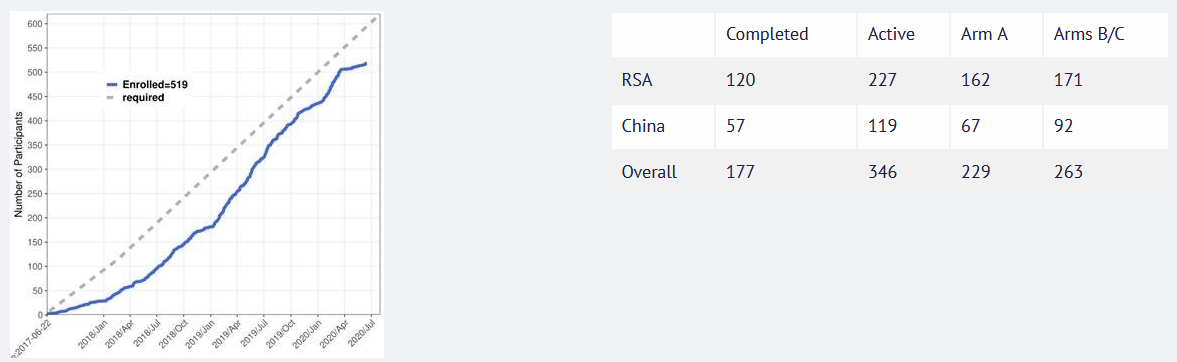
The Chinese PredictTB test sites are currently recruiting. Assuming that the patient flow at the South African sites is not too negatively affected by COVID-19 in the following months, enrolling the remaining circa one hundred patients needed to reach the project target by the end of this year should be possible, according to Bronwyn Smith.
COVID-19 opens new doors for additional research as part of PredictTB
The coronavirus outbreak poses several challenges for the PredictTB team, the most pressing ones being: potential lack of money necessary to complete the recruitments towards the end of this year; shortage of time towards the end of the project as follow-ups will have to run longer as a result of the prolonged recruitment process; and the current challenge in getting access to sufficient PPE at all test sights.
All participants at the South African PredictTB test sites will be offered both a TB and a COVID-19 test when they are screened for eligibility. This opens new doors for additional research as a part of PredictTB. With COVID-19 as a new contributor, the PredictTB team will need to include analysis to find out how biomarkers of interest for TB are affected when participants are co-infected with COVID-19.
Free COVID-19 testing for high-risk PredictTB trial patients
Apart from offering free COVID-19 testing to eligible new recruits, free COVID-19 testing will also be offered to patients who are already on the study and who develop worsening symptoms of TB through the course of the study.
Already ongoing studies continued during the lockdown
PredictTB test sites have worked hard in the past months to ensure that studies with previously enrolled patients were able to continue during the lockdown while adhering to the highest safety standards and government regulations. Provisions included cutting back on the number of face-to-face visits with the patients and replaced them with telephone meetings.
